Dickinson, R., White,
I., Lieb, W.R. and Franks, N.P. 2000. Stereoselective loss of righting reflex in rats
by isoflurane, Anesthesiology, v93, 837-843.
Hall, A.C., Griffith, T.N., Tsikolia, M., Kotey, F.O., Gill, N., Humbert, D.J., Watt, E.E., Yermolina, Y.A., Goel, S., El-Ghendy, B., Hall, C.D. (2011). Cyclohexanol analogues are positive modulators of GABAA receptor currents and act as general anesthetics in vivo. European Journal of Pharmacology, 667, 175-181. Campbell, L.L., Tyson, J.A., Stackpole, E.E., Hokenson, K.E., Sherrill, H., McKeon, J.E., Kam, S.A., Edmands, S.D., Suarez, C., Hall, A.C. (2011). Assessment of general anesthetic cytotoxicity in murine cortical neurones in dissociated culture. Toxicology, 283, 1-7. Tsikolia M., Hall A.C., Suarez C., Nylander Z.O., Wardlaw S.M., Gibson M.E., Valentine K.L., Onyewadume L.N., Ahove D.A., Woodbury M., Mongare M.M., Hall C.D., Wang Z., Draghici B. and Katritzky A. R. (2009) Synthesis and characterization of a redox-active ion channel supporting cation flux in lipid bilayer. Journal of Organic and Biomolecular Chemistry, 7, 3862-3870. Edmands, S.D. and Hall, A.C. (2009) Metallothioneins are essential for isoflurane preconditioning in murine cortical cultures.Anesthesiology 110, 538-547.
Watt, E.E. Betts, B.A.Kotey, F.O., Humbert D.J., Griffith T.N., Kelly E.W., Jenkins, A. and Hall A.C. (2008). Menthol shares general anesthetic activiy and sites of action on the GABAA receptor with the intravenous anesthetic propofol. European Journal of Pharmacology, 590, 120-126.
Hall, A.C., Stevens, R.J.N., Betts, B.A., Yeung W-Y., Kelley, J.C., Harrison, N.L. (2005). Subunit-dependent block by isoflurane of wild-type and mutant a1 S270H GABAA receptor currents in Xenopus oocytes. Neuroscience Letters, 382, 332-337.
Hall, A.C., Turcotte, C.M., Betts, B.A., Yeung, W-Y., Agyeman, A..S. and Burk, L.A. (2004). Modulation of Human GABAA and glycine receptor currents by menthol and related monoterpenoids. European Journal of Pharmacology, 506, 9-16.
Hall, A.C., Rowan, K.C., Stevens, R.J.N., Kelley, J.C., Harrison, N.L. (2004). The effects of isoflurane on desensitized wild-type and a1(S270H) g-aminobutyric acid type A receptors . Anesthesia and Analgesia, 98: 1297-1304.. Hall, A.C. and M.E. Harrington (2003) ‘Experimental Methods in Neuroscience’: an undergraduate neuroscience laboratory course for teaching data collection, statistical analyses and report writing. Journal of Undergraduate Neuroscience Education, 2, A1-7.
Hall, A.C., Suarez, C., Hom-Choudhory, A., Manu, A.N.A., Hall, C.D., Kirkovits, G.J., Ghiriviga, I. (2003). Cation transport by a redox-active synthetic ion channel. Organic and Biomolecular Chemistry, 1, 2973-2982. Dickinson, R., Sousa, S.L.M., Hall, A.C., Lieb, W.R. & Franks, N.P., (2000). Is the NMDA Receptor a Molecular Target for the “Inert” Gas Xenon? Progress in Anesthetic Mechanisms, 6, 389-394
Hall, A.C., Earle-Cruikshanks, G. and Harrington, M.E. (1999). Role of membrane conductances and protein synthesis in subjective day phase advances of the hamster circadian clock by neuropeptide Y. European Journal of Neuroscience, 11, 3424-3432 Diaz-Munoz, M., Dent, M.A.R., Granados-Fuentes, D., Hall, A.C., Hernandez-Cruz, A., Harrington, M.E., Aguilar-Roblero, R. (1999). Circadian rhythm of expression of the ryanodine receptor type 2 in neurons from the suprachiasmatic nuclei of rodents. Neuroreport, 10, 481-486. Harrington, M.E., Hoque, S., Hall, A.C., Golombek, D. and Biello, S.M. (1999). Pituitary adenylate ctclase activating peptide phase shifts circadian rhythms in a manner similar to light. Journal of Neuroscience, 19, 6637-6642 Hall, C.D., Kirkovits, G.J. and Hall, A.C. (1999) . Towards a redox ion channel. Chemical Communications, 18 1897-1898. Meyer, J.L., Hall, A.C. and Harrington, M.E. (1998). Histamine phase shifts the hamster circadian pacemaker via a NMDA-dependent mechanism. Journal of Biological Rhythms, 13, 288-295. Hall, C.D., Lowther, N., Tweedy, B.R., Hall, A.C. and Shaw, G. (1998). The kinetics and mechanism of the phosphorus-catalysed dimerisation of acrylonitrile. Journal of Chemical Society, Perkin 2 2047-2054. Franks, N.P., Dickinson, R., Sousa, S.L.M., Hall, A.C. and Lieb, W.R. (1998). How does the ‘inert’ gas xenon produce general anaesthesia? Nature, 396, 324 Hall, A.C., Hoffmaster, R.M., Stern, E.L., Harrington, M.E. and Bickar, D. (1997). Suprachiasmatic nucleus neurons are glucose-sensitive. Journal of Biological Rhythms, 12, 388-400. Lee, K., Khan, R.N., Rowe, I.C.M., Ozanne, S.E., Hall, A.C., Papadakis, E., Hales, C.N. and Ashford, M.L.J. (1996). Ciclazindol inhibits ATP-sensitive K+ channels and stimulates insulin secretion in CRI-G1 insulin-secreting cells. Molecular Pharmacology, 49, 715-721.
Downie, D.L., Hall, A.C., Lieb, W.R. and Franks, N.P. (1996). Effects of inhalational general anaesthetics on native glycine receptors in rat medullary neurones and recombinant glycine receptors in Xenopus oocytes. British Journal of Pharmacology, 118, 493-502.
Owen, D.G., Hall, A.C., Stephens, G., Stow, J. and Robertson, B. (1996). The relative potencies of dendrotoxins as blockers of the cloned voltage-gated K+ channel, mKv1.1 (MK-1), when stably expressed in Chinese hamster ovary cells. British Journal of Pharmacology, 120,1029-1034.
Hall, A.C., Stow, J., Sorensen, R., Dolly, J.O. and Owen, D.G. (1994). Blockade by dendrotoxin homologues of voltage-dependent K+ currents in cultured sensory neurones from neonatal rats. British Journal of Pharmacology, 113, 959-967. Hall, A.C., Lieb, W.R. and Franks, N.P. (1994) Insensitivity of P-type calcium channels to inhalational and intravenous general anesthetics. Anesthesiology, 81, 117-123. Hall, A.C., Lieb, W.R. and Franks, N.P. (1994) Stereoselective and non-stereoselective actions of isoflurane on the GABAA receptor. British Journal of Pharmacology, 112, 906-910. Dolly, J.O., Muniz, Z.M., Parcej, D.N., Hall, A.C., Scott, V.E.S., Awan, K.A. and Owen, D. (1994). Subtypes of fast-activating, voltage-gated K+ channels in the nervous system. In: Neurotoxins and Neurobiology (eds. Tipton, K.F. & Dajas, F.), Ellis Horwood series in Neuroscience, pp. 103-122. Hall, A.C., Tibbs, G.R., Dolly, J.O., Lieb, W.R. and Franks, N.P. (1993). A simple method for recording single-channel activity from synaptic plasma membranes. Journal of Neuroscience Methods, 49, 81-91. Back to top> Edmands, S. D., Hall, A.C. (2011) A role for metallothioneins I + II as molecular sensors of oxidative stress in anesthetic preconditioning, Abstr. A-303-0021-01046 XXVth International Symposium on Cerebral Blood Flow, Metabolism and Function, Barcelona, Spain. Hall A.C., Edmands, S. D. (2011) Metallothioneins I + II act as sensors of oxidative stress in isoflurane-mediated preconditioning of cultured neurones, Proceedings of the British Neuroscience Association, Harrogate, UK Ziemba A.M., Flynn E.R., Hawkins B., Edmands, S.D., Hall A.C., Investigation of a Molecular Mechanism of Anesthetic Preconditioning. (2011) Faculty for undergraduate Neuroscience Meeting, Society for Neuroscience Abstracts, Washington DC.. Nyitrai, H., Ziemba, A.M., Campbell, L-L., Rodes, J., Croft, C.J., Nhundu, B.J., Hall, A.C. General Anesthetic‐Induced Neurite Retraction in Cultured Murine Cortical Neurons. (2010) Faculty for undergraduate Neuroscience Meeting, Society for Neuroscience Abstracts, San Diego, CA. Edmands S.D., Campbell L-L and Hall, A.C. Role for Metallothioneins I + II as sensors of oxidative stress in isoflurane-mediated anesthetic preconditioning in vitro (2009) Society for Neuroscience Abstracts, Chicago, IL.
Edmands, S.D. and Hall A.C. Metallothioneins I/II are required for isoflurane preconditioning in murine cortical cultures. (2009) 20th British Neuroscience Association Abstracts 62.02, Liverpool, UK.
Hall, A.C. and Edmands, S.D. Metallothioneins I/II are required for isoflurane preconditioning in murine cortical cultures. (2008) 6th FENS forum of European Neuroscience, Geneva, Switzerland.
Tyson, J.A., Campbell, L-L., Stackpole E.E., Edmands S.D. and Hall, A.C. Assessment of neurotoxicity by anesthetic cocktails on primary cultures from dissociated neonatal murine cortex. (2008) Society for Neuroscience Abstracts, Washington, DC.
Griffith T.N, .Humbert D.J., Yermolina Y.A., Kotey F.O.,and Hall A.C. Positive modulation of GABA-A receptor currents and anesthesia by cyclohexanol analogs (2007) Society for Neuroscience Abstracts, San Diego, CA.
Stackpole, E.E., Hokenson, K.E., McKeon, J.E. and Hall, A.C. Assessment of neurotoxicity of anesthetic cocktails on primary cultures of dissociated murine cortical neurones. (2007) British Neuroscience Association Abstracts 66.01, Harrogate UK. Edmands, S.D., Hall, A.C. Metallothioneins I and II mediate protective effect of isoflurane-induced anesthetic preconditioning in vitro (2006) Society for Neuroscience Abstracts, 36, 187.1 Edmands, S.D., Hall, A.C. (2005) DNA microarray analysis of isoflurane induced differential gene expression in rat liver, kidney, and heart. American Society of Anesthesiologists Meeting, Atlanta GA. Betts B.A., Yeung W-Y., Agyeman A.S., Turcotte C.M., Burk L.A., Hall, A.C.. (2005). Modulation of Human GABAA and Glycine Receptor Currents by Menthol and Related Monoterpenoids. Departments of Biological Sciences and Chemistry, Smith College, Northampton, MA 01062, USA. Society for Neuroscience Abstracts, 35, 488.17 Harrington M.E., Powell B., Hall A.C. (2005). Teaching Research Skills to Students in Neuroscience, Neuroscience Program, Depts. Psychology and Biological Sciences, Smith College, Northampton, MA 01063, USA. Society for Neuroscience Abstracts, 35, 20.13. LaDow, E.S., Edmands, S.D., Hall, A.C. (2004). Differential gene expression in a mouse neuroblastoma cell line after exposure to the volatile anesthetic isoflurane. Society for Neuroscience Abstracts, 34, 343.9. Sandstrom, N.J., Turgeon S.M., George S.A.., Thornton, J.A., Smith D.A., Hall A.C., Harrington, M.E. (2004). A Comparison of Neuroscience Programs at Amherst, Oberlin, Smith and Williams Colleges. Society for Neuroscience Abstracts, 34, 28.17. Betts, B.A. Agyeman,, S.A., Turcotte, C.M., Yeung, W-Y., Burk, L.A.. and Hall, A.C. (2004) Modulation of Human GABAA and Glycine Receptors Currents by Monoterpenoids. N.E.U.R.O.N. Meeting, Wheaton College, MA. Scordilis,S.P., Aung, L.L., Hall A.C. (2004) Global gene expression during unweighting and unloading. IBE Meeting, Austin TX. Stevens, R.J.N., Rowan, K.C., Turcotte, C.M. and Hall, A.C. (2003) Isoflurane relieves slow desensitization of GABAA receptors expressed in Xenopus ooctyes. Society for Neuroscience Abstracts, 33, 48.8.
Hall, A.C. (2002). ‘Experimental methods in Neuroscience’: a new undergraduate behavioral neuroscience laboratory course for teaching data collection, statistical analyses and report writing. Society for Neuroscience Abstracts, 32, 22.31. Stevens, R.J.N., Rowan, K.C., Turcotte, C.M. and Hall, A.C. (2002) Effects of the Volatile Anesthetic Isoflurane on Desensitized Human GABAA Receptors Expressed in Xenopus oocytes. MIT and Harvard Hippocratic Society Meeting.
Meyer, J.L., Hall, A.C. and Harrington, M.E. (1998). Histamine phase shifts the hamster circadian pacemaker via a NMDA-dependent mechanism. VIth Meeting Society for Research on Biological Rhythms, P187.
Hall, A.C. (1998). In vitro function of NPY receptors in the SCN. Proceedings of the FASEB Meeting, ‘Neurobiology of vertebrate circadian rhythms’, Snowmass, CO. Hall, A.C. and Harrington, M.E. (1997). Neuropeptide Y activates ATP-sensitive K+ channels in hamster suprachiasmatic nucleus neurons: an essential step for non-photic phase advances in vitro. Gordon Conference for Chronobiology, New London, NH. Hall, A.C. and Harrington, M.E. (1997). ATP-sensitive K+ channels and glucose-sensitivity in hamster SCN neurons. Society for Neuroscience Abstracts, 23, 99.9. Lei, C, Hall, A.C. and Powell, J.A. (1997). The control of triad-associated protein expression by the a1-subunit of the dihydropyridine receptor (DHPR) in skeletal muscle in culture. Biophysical Society Meeting, A378, P177.
Hall, A.C. and Harrington, M.E. (1996). Neuropeptide Y activates large conductance channels in suprachiasmatic nucleus (SCN) neurons. Society for Neuroscience Abstracts, 22, 808.5. Lee, K., Khan, R.N., Rowe, I.C.M., Hall, A.C. and Ashford, M.L.J. (1995). Inhibition of KATP currents by ciclazindol in the CRI-G1 insulin-secreting cell line. Proceedings of the British Pharmacological Society, P92. Hall, A.C., Downie, D.L., Dickinson, R., Tomlin, S.L., Franks, N.P. and Lieb, W.R. (1995). Action of the volatile anesthetic isoflurane on neuronal & cloned glycine receptors. Society for Neuroscience Abstracts, 21, 634.5. Ozanne, S.E., Lee, K., Hall, A.C., Rowe, I.C.M. Ashford, M.L.J. and Hales, C.N. (1994). Ciclazindol inhibits the ATP-sensitive potassium channel in the CRI-G1 insulin-secreting cell line. Diabetic Medicine, 11 (Supp. 1), P71. Tippins, J.R., Hall, A.C., Bowen, P.R. and Dolly, J.O. (1994). In vitro cardiovascular effects of Toxin I from Dendroaspis polylepis polylepis. British Journal of Pharmacology, 111, 243P. Owen, D.G., Robertson, B., Hall, A.C. and Scott, J.A. (1992). Blockade by peptidic toxins of MK1 potassium currents in CHO cells. Society for Neuroscience Abstracts, 18, 335.1.
Hall, A.C., Stow, J., Dolly, J.O. and Owen, D.G. (1991). Dendrotoxin homologues block voltage-dependent potassium currents in cultured rat dorsal root ganglion cells by different mechanisms. 10th World Congress on Animal, Plant and Microbial Toxins, Abstract 371. Owen, D.G., Hall, A.C., Sorensen, R.G. and Stow, J. (1990). Inhibition of outward currents in dorsal root ganglion cells by dendrotoxin homologues. Society for Neuroscience Abstracts, 16, 156.8. |
Copyright © 2006 Smith College, Northampton, MA 01096 | Last updated June 18, 2012
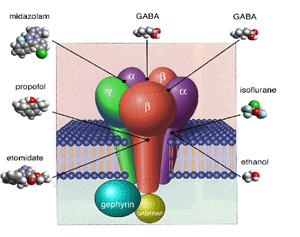
 It
is now widely accepted that volatile anesthetics mediate their actions through direct interaction
with membrane proteins rather than lipids (Franks and Lieb, 1998). Many neuronal ion channels
are now recognized as likely candidate targets for anesthetic agents. At clinical concentrations,
ligand-gated ion channels (e.g. GABA A, Glycine) are typically more sensitive to modulation
by anesthetics (see Hall et al., 1994b) than the voltage gated family (see Hall et
al., 1994a). Furthermore, it has been shown for ligand-gated channels that there are differential
sensitivities to general anesthetics depending on the receptor subunit combinations that are
expressed (Violet et al., 1997).
It
is now widely accepted that volatile anesthetics mediate their actions through direct interaction
with membrane proteins rather than lipids (Franks and Lieb, 1998). Many neuronal ion channels
are now recognized as likely candidate targets for anesthetic agents. At clinical concentrations,
ligand-gated ion channels (e.g. GABA A, Glycine) are typically more sensitive to modulation
by anesthetics (see Hall et al., 1994b) than the voltage gated family (see Hall et
al., 1994a). Furthermore, it has been shown for ligand-gated channels that there are differential
sensitivities to general anesthetics depending on the receptor subunit combinations that are
expressed (Violet et al., 1997).